Table of Contents
1. Thermodynamic Properties
1.1 Thermodynamic properties and other basic concepts
1.2 Measured thermodynamic properties
1.3 Equilibrium
1.4 States and phases
1.5 Thermodynamic property tables
1.6 Summary
1.7 Problems
2. The First Law of Thermodynamics
2.1 First law of thermodynamics
2.2 Processes and paths
2.3 First law of thermodynamics for closed systems
2.4 First law of thermodynamics for open systems
2.5 Thermochemical data for \(U\) and \(H\)
2.6 Reversible processes in closed systems
2.7 Open system energy balances on process equipment
2.8 Summary
2.9 Problems
2.10 Problems (continued)
2.11 Problems (continued)
2.12 Problems (continued)
2.13 Problems (continued)
2.14 Problems (continued)
3. Entropy and The Second Law of Thermodynamics
3.1 Directionality of processes/spontaneity
3.2 Reversible and irreversible processes and their relationship to directionality
3.3 Entropy, the thermodynamic property
3.4 The second law of thermodynamics
3.5 Molecular view of entropy
3.6 The second law of thermodynamics for closed and open systems
3.7 Calculation of \(s\) for an ideal gas
3.8 The mechanical energy balance and the Bernoulli equation
3.9 Vapor-compression power and refrigeration cycles
3.10 Exergy (availability) analysis
3.11 Summary
3.12 Problems
3.13 Problems (continued)
3.14 Problems (continued)
3.15 Problems (continued)
3.16 Problems (continued)
3.17 Problems (continued)
4. Equations of State and Intermolecular Interactions
4.1 Equations of State
4.2 Intermolecular interactions
4.3 Potential functions and chemical effects
4.4 Cubic equations of state
4.5 Virial equation of state
4.6 Equations of state for liquids and solids
4.7 Generalized compressibility charts
4.8 Mixing rules
4.9 Summary
4.10 Problems
4.11 Problems (continued)
4.12 Problems (continued)
5. The Thermodynamic Web
5.1 Types of thermodynamic properties
5.2 Thermodynamic property relations
5.3 Calculation of fundamental and derived properties using equations of state and other measured properties
5.4 Departure functions
5.5 Joule-Thompson Expansion and Liquefaction
5.6 Summary
5.7 Problems
5.8 Problems (continued)
5.9 Problems (continued)
5.10 Problems (continued)
5.11 Problems (continued)
6. Phase Equilibria I: Problem Formulation
6.1 The phase equilibria problem
6.2 Pure species phase equilibrium: Gibbs Energy
6.3 Pure species phase equilibrium: Relations between P and T
6.4 Thermodynamics of mixing
6.5 Partial molar properties
6.6 The chemical potential
6.7 Summary
6.8 Problems
6.9 Problems (continued)
6.10 Problems (continued)
6.11 Problems (continued)
7. Phase Equilibria II: Fugacity
7.1 Introducing fugacity
7.2 Fugacity in the vapor phase: Pure species
7.3 Fugacity in the vapor phase: Mixtures
7.4 Fugacity in the liquid phase: Reference states and activity coefficients
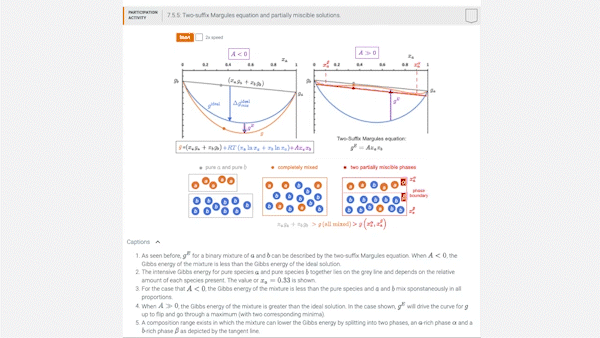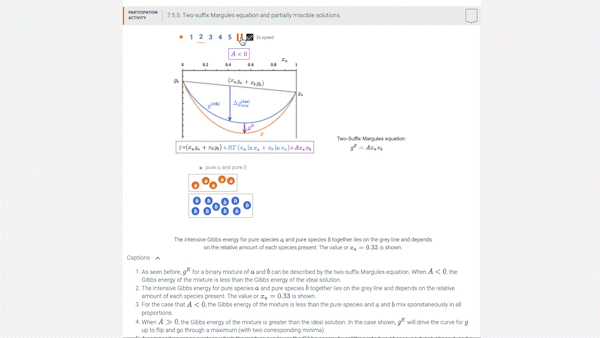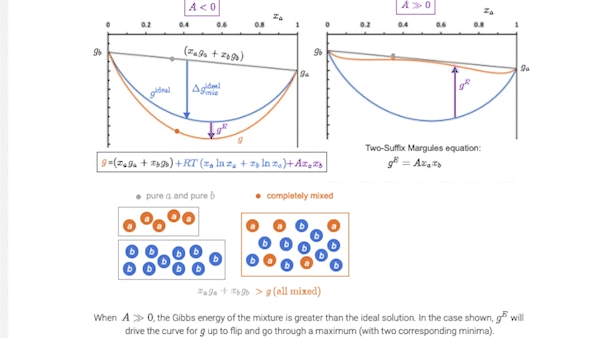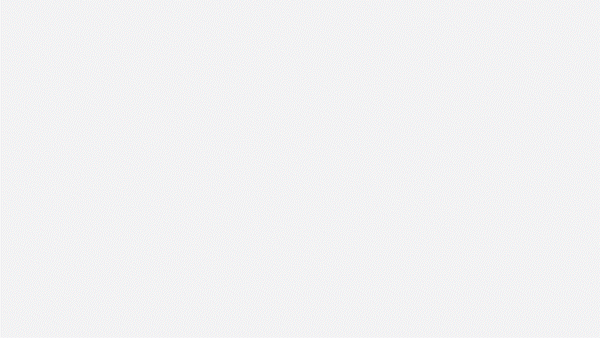
7.5 Introducing models for \(g^E\): the two-suffix Margules equation
7.6 Models for \(g^E\): Binary mixtures
7.7 Temperature and pressure dependence of \(g^E\)
7.8 Models for \(g^E\): Multicomponent mixtures
7.9 Summary
7.10 Problems
7.11 Problems (continued)
7.12 Problems (continued)
7.13 Problems (continued)
7.14 Problems (continued)
8. Phase Equilibria III: Applications
8.1 Vapor-liquid equilibrium (VLE): Concepts and phase diagrams
8.2 VLE: Bubble point, dew point and the lever rule
8.3 VLE: Solubility of gases in liquids
8.4 VLE: Fitting models for \(g^E\) with VLE data
8.5 VLE: Equation of state method
8.6 LLE: Liquid-liquid equilibrium
8.7 VLLE: Vapor-liquid-liquid equilibrium
8.8 SLE and SSE: Solid-liquid and solid-solid equilibrium
8.9 Colligative Properties
8.10 Summary
8.11 Problems
8.12 Problems (continued)
8.13 Problems (continued)
8.14 Problems (continued)
8.15 Problems (continued)
8.16 Problems (continued)
9. Chemical Reaction Equilibria
9.1 Chemical reactions: Kinetics and thermodynamics
9.2 Equilibrium constant formulation
9.3 Using thermochemical data to calculate \(K\)
9.4 Chemical equilibrium for a single reaction
9.5 Multiple chemical reactions
9.6 Equilibrium in electrochemical processes
9.7 Reaction equilibria of point defects in crystalline solids
9.8 Summary
9.9 Problems
9.10 Problems (continued)
9.11 Problems (continued)
9.12 Problems (continued)
9.13 Problems (continued)
10. Appendix: Physical Constants, Conversion Factors, and Nomenclature
10.1 Physical constants, conversion factors, and nomenclature
10.2 Periodic Table of the Elements
11. Appendix: Physical Property Data
11.1 Critical constants, acentric factors, and antoine coefficients
11.2 Heat capacity data
11.3 Enthalpy and Gibbs Energy of Formation at 298 K and 1 bar
12. Appendix: Steam Tables
12.1 Symbols used in the steam tables
12.2 Saturated water: temperature table
12.3 Saturated water: pressure table
12.4 Saturated water: solid-vapor
12.5 Superheated water vapor
12.6 Subcooled liquid water
13. Appendix: Lee-Kesler Generalized Correlation Tables
13.1 Values for \(z^{(0)}\)
13.2 Values for \(z^{(1)}\)
13.3 Values for \(\left[\frac{h_{T_{r}, P_{r}}-h_{T_{r}, P_{r}}^{\text {ideal gas }}}{R T_{c}}\right]^{(0)}\)
13.4 Values for \(\left[\frac{h_{T_{r}, P_{r}}-h_{T_{r}, P_{r}}^{\text {ideal gas }}}{R T_{c}}\right]^{(1)}\)
13.5 Values for \(\left[\frac{s_{T_{r}, P_{r}}-s_{T_{r}, P_{r}}^{\text {ideal gas }}}{R}\right]^{(0)}\)
13.6 Values for \(\left[\frac{s_{T_{r}, P_{r}}-s_{T_{r}, P_{r}}^{\text {ideal gas }}}{R}\right]^{(1)}\)
13.7 Values for log \(\left [ \Phi ^{(0)} \right ]\)
13.8 Values for log \(\left [ \Phi ^{(1)} \right ]\)
14. Appendix: Unit Systems
14.1 Common variables used in thermodynamics and their associated units
14.2 Conversion between CGS (gaussian) units and SI units
15. Appendix: ThermoSolver Software
15.1 Software Description
15.2 Corresponding States Using The Lee–Kesler Equation of State
Same Text, More Action
Based on the original textbook, Engineering and Chemical Thermodynamics helps students understand and visualize thermodynamics through a qualitative discussion of the role of molecular interactions and a highly visual presentation of the material. In this zyVersion you’ll find:
- Over 150 animations that allow students to watch molecules interact
- Over 500 participation questions which help to break down complex concepts
- Newly added Challenge Activities (“auto-graded homework problems”)
Optional Challenge Activities
As an instructor, you have the ability to mark Challenge Activities as optional, which means you can indicate which Challenge Activities your students need to complete in order to meet your course requirements. Those marked as optional won’t contribute to point totals in reports or assignments.
What is a zyVersion?
zyVersions are leading print titles converted and adapted to zyBooks’ interactive learning platform, allowing for a quick and easy transition to an engaging digital experience for instructors and students.
zyBooks’ web-native content helps students visualize concepts to learn faster and more effectively than with a traditional textbook.
This zyVersion of Engineering and Chemical Thermodynamics benefits both students and instructors:
- Instructor benefits
- Customize your course by reorganizing existing content or adding your own content
- Continuous publication model updates your course with the latest content and technologies
- Robust reporting gives you insight into students’ progress, reading and participation
- Student benefits
- Learning questions and other content serve as an interactive form of reading and provide instant feedback
- Concepts come to life through extensive animations embedded into the interactive content
- Save chapters as PDFs to reference material at any time, even after course completion
Authors
Milo D. Koretsky
Professor of Chemical and Biological Engineering, Professor of Education, McDonnell Family Bridge Professorship, Tufts University
Matthew Liberatore
Professor of Chemical Engineering, University of Toledo
Contributor
Nikitha Sambamurthy
Editorial Director / zyBooks