Table of Contents
1. Introduction
1.1 Historical perspective
1.2 Materials science and engineering
1.3 Why study materials science and engineering?
1.4 Classification of materials
1.5 Advanced materials
1.6 Modern materials’ needs
1.7 Summary
1.8 References
1.9 Questions and problems
2. Atomic Structure and Interatomic Bonding
2.1 Introduction
2.2 Atomic structure: Fundamental concepts
2.3 Atomic structure: Electrons in atoms
2.4 Atomic structure: The periodic table
2.5 Atomic bonding in solids: Bonding forces and energies
2.6 Atomic bonding in solids: Primary interatomic bonds
2.7 Atomic bonding in solids: Secondary bonding or van der Waals bonding
2.8 Atomic bonding in solids: Mixed bonding
2.9 Atomic bonding in solids: Molecules
2.10 Atomic bonding in solids: Bonding type-material classification correlations
2.11 Summary
2.12 Equation summary
2.13 List of symbols
2.14 References
2.15 Questions and problems
3. Structures of Metals and Ceramics
3.1 Introduction
3.2 Crystal structures: Fundamental concepts
3.3 Crystal structures: Unit cells
3.4 Crystal structures: Metallic crystal structures
3.5 Crystal structures: Density computations—metals
3.6 Crystal structures: Ceramic crystal structures
3.7 Crystal structures: Density computations—ceramics
3.8 Crystal structures: Silicate ceramics
3.9 Crystal structures: Carbon
3.10 Crystal structures: Polymorphism and allotropy
3.11 Crystal structures: Crystal systems
3.12 Crystallographic points, directions, and planes: Point coordinates
3.13 Crystallographic points, directions, and planes: Crystallographic directions
3.14 Crystallographic points, directions, and planes: Crystallographic planes
3.15 Crystallographic points, directions, and planes: Linear and planar densities
3.16 Crystallographic points, directions, and planes: Close-packed crystal structures
3.17 Crystalline and noncrystalline materials: Single crystals
3.18 Crystalline and noncrystalline materials: Polycrystalline materials
3.19 Crystalline and noncrystalline materials: Anisotropy
3.20 Crystalline and noncrystalline materials: X-ray diffraction: Determination of crystal structures
3.21 Crystalline and noncrystalline materials: Noncrystalline solids
3.22 Summary
3.23 Equation summary
3.24 List of symbols
3.25 References
3.26 Questions and problems
4. Polymer Structures
4.1 Introduction
4.2 Hydrocarbon molecules
4.3 Polymer molecules
4.4 The chemistry of polymer molecules
4.5 Molecular weight
4.6 Molecular shape
4.7 Molecular structure
4.8 Molecular configurations
4.9 Thermoplastic and thermosetting polymers
4.10 Copolymers
4.11 Polymer crystallinity
4.12 Polymer crystals
4.13 Summary
4.14 Equation summary
4.15 List of symbols
4.16 References
4.17 Questions and problems
5. Imperfections in Solids
5.1 Introduction
5.2 Point defects: Point defects in metals
5.3 Point defects: Point defects in ceramics
5.4 Point defects: Impurities in solids
5.5 Point defects: Point defects in polymers
5.6 Specification of composition
5.7 Miscellaneous imperfections: Dislocations—linear defects
5.8 Miscellaneous imperfections: Interfacial defects
5.9 Miscellaneous imperfections: Bulk or volume defects
5.10 Miscellaneous imperfections: Atomic vibrations
5.11 Microscopic examination: Basic concepts of microscopy
5.12 Microscopic examination: Microscopic techniques
5.13 Microscopic examination: Grain-size determination
5.14 Summary
5.15 Equation summary
5.16 List of symbols
5.17 References
5.18 Questions and problems
6. Diffusion
6.1 Introduction
6.2 Diffusion mechanisms
6.3 Fick’s first law
6.4 Fick’s second law—nonsteady-state diffusion
6.5 Factors that influence diffusion
6.6 Diffusion in semiconducting materials
6.7 Other diffusion paths
6.8 Diffusion in ionic and polymeric materials
6.9 Summary
6.10 Equation summary
6.11 List of symbols
6.12 References
6.13 Questions and problems
7. Mechanical Properties
7.1 Introduction
7.2 Concepts of stress and strain
7.3 Elastic deformation: Stress-strain behavior
7.4 Elastic deformation: Anelasticity
7.5 Elastic deformation: Elastic properties of materials
7.6 Mechanical behavior—metals: Tensile properties
7.7 Mechanical behavior—metals: True stress and strain
7.8 Mechanical behavior—metals: Elastic recovery after plastic deformation
7.9 Mechanical behavior—metals: Compressive, shear, and torsional deformations
7.10 Mechanical behavior—ceramics: Flexural strength
7.11 Mechanical behavior—ceramics: Elastic behavior
7.12 Mechanical behavior—ceramics: Influence of porosity on the mechanical properties of ceramics
7.13 Mechanical behavior—polymers: Stress-strain behavior
7.14 Mechanical behavior—polymers: Macroscopic deformation
7.15 Mechanical behavior—polymers: Viscoelastic deformation
7.16 Other mechanical property considerations: Hardness
7.17 Other mechanical property considerations: Hardness of ceramic materials
7.18 Other mechanical property considerations: Tear strength and hardness of polymers
7.19 Property variability and design/safety factors: Variability of material properties
7.20 Property variability and design/safety factors: Design/safety factors
7.21 Summary
7.22 Equation summary
7.23 List of symbols
7.24 References
7.25 Questions and problems
8. Deformation and Strengthening Mechanisms
8.1 Introduction
8.2 Deformation mechanisms for metals: Historical
8.3 Deformation mechanisms for metals: Basic concepts of dislocations
8.4 Deformation mechanisms for metals: Characteristics of dislocations
8.5 Deformation mechanisms for metals: Slip systems
8.6 Deformation mechanisms for metals: Slip in single crystals
8.7 Deformation mechanisms for metals: Plastic deformation of polycrystalline metals
8.8 Deformation mechanisms for metals: Deformation by twinning
8.9 Mechanisms of strengthening in metals: Strengthening by grain size reduction
8.10 Mechanisms of strengthening in metals: Solid-solution strengthening
8.11 Mechanisms of strengthening in metals: Strain hardening
8.12 Recovery, recrystallization, and grain growth: Recovery
8.13 Recovery, recrystallization, and grain growth: Recrystallization
8.14 Recovery, recrystallization, and grain growth: Grain growth
8.15 Deformation mechanisms for ceramic materials: Crystalline ceramics
8.16 Deformation mechanisms for ceramic materials: Noncrystalline ceramics
8.17 Mechanisms of deformation and for strengthening of polymers: Deformation of semicrystalline polymers
8.18 Mechanisms of deformation and for strengthening of polymers: Factors that influence the mechanical properties of semicrystalline polymers
8.19 Mechanisms of deformation and for strengthening of polymers: Deformation of elastomers
8.20 Summary
8.21 Equation summary
8.22 List of symbols
8.23 References
8.24 Questions and problems
9. Failure
9.1 Introduction
9.2 Fracture: Fundamentals of fracture
9.3 Fracture: Ductile fracture
9.4 Fracture: Brittle fracture
9.5 Fracture: Principles of fracture mechanics
9.6 Fracture: Brittle fracture of ceramics
9.7 Fracture: Fracture of polymers
9.8 Fracture: Fracture toughness testing
9.9 Fatigue: Cyclic stresses
9.10 Fatigue: The S-N curve
9.11 Fatigue: Fatigue in polymeric materials
9.12 Fatigue: Crack initiation and propagation
9.13 Fatigue: Factors that affect fatigue life
9.14 Fatigue: Environmental effects
9.15 Creep: Generalized creep behavior
9.16 Creep: Stress and temperature effects
9.17 Creep: Data extrapolation methods
9.18 Creep: Alloys for high-temperature use
9.19 Creep: Creep in ceramic and polymeric materials
9.20 Summary
9.21 Equation summary
9.22 List of symbols
9.23 References
9.24 Questions and problems
10. Phase Diagrams
10.1 Introduction
10.2 Definitions and basic concepts: Solubility limit
10.3 Definitions and basic concepts: Phases
10.4 Definitions and basic concepts: Microstructure
10.5 Definitions and basic concepts: Phase equilibria
10.6 Definitions and basic concepts: One-component (or unary) phase diagrams
10.7 Binary phase diagrams: Binary isomorphous systems
10.8 Binary phase diagrams: Interpretation of phase diagrams
10.9 Binary phase diagrams: Development of microstructure in isomorphous alloys
10.10 Binary phase diagrams: Mechanical properties of isomorphous alloys
10.11 Binary phase diagrams: Binary eutectic systems
10.12 Binary phase diagrams: Development of microstructure in eutectic alloys
10.13 Binary phase diagrams: Equilibrium diagrams having intermediate phases or compounds
10.14 Binary phase diagrams: Eutectoid and peritectic reactions
10.15 Binary phase diagrams: Congruent phase transformations
10.16 Binary phase diagrams: Ceramic phase diagrams
10.17 Ternary phase diagrams
10.18 The Gibbs phase rule
10.19 The iron-carbon system: The iron-iron carbide (Fe-Fe₃C) phase diagram
10.20 The iron-carbon system: Development of microstructure in iron-carbon alloys
10.21 The iron-carbon system: The influence of other alloying elements
10.22 Summary
10.23 Equation summary
10.24 List of symbols
10.25 References
10.26 Questions and problems
11. Phase Transformations
11.1 Introduction
11.2 Phase transformations in metals: Basic concepts
11.3 Phase transformations in metals: The kinetics of phase transformations
11.4 Phase transformations in metals: Metastable versus equilibrium states
11.5 Microstructural and property changes in iron-carbon alloys: Isothermal transformation diagrams
11.6 Microstructural and property changes in iron-carbon alloys: Continuous-cooling transformation diagrams
11.7 Microstructural and property changes in iron-carbon alloys: Mechanical behavior of iron-carbon alloys
11.8 Microstructural and property changes in iron-carbon alloys: Tempered martensite
11.9 Microstructural and property changes in iron-carbon alloys: Review of phase transformations and mechanical properties for iron-carbon alloys
11.10 Precipitation hardening: Heat treatments
11.11 Precipitation hardening: Mechanism of hardening
11.12 Precipitation hardening: Miscellaneous considerations
11.13 Crystallization, melting, and glass transition phenomena in polymers: Crystallization
11.14 Crystallization, melting, and glass transition phenomena in polymers: Melting
11.15 Crystallization, melting, and glass transition phenomena in polymers: The glass transition
11.16 Crystallization, melting, and glass transition phenomena in polymers: Melting and glass transition temperatures
11.17 Crystallization, melting, and glass transition phenomena in polymers: Factors that influence melting and glass transition temperatures
11.18 Summary
11.19 Equation summary
11.20 List of symbols
11.21 References
11.22 Questions and problems
12. Electrical Properties
12.1 Introduction
12.2 Electrical conduction: Ohm’s law
12.3 Electrical conduction: Electrical conductivity
12.4 Electrical conduction: Electronic and ionic conduction
12.5 Electrical conduction: Energy band structures in solids
12.6 Electrical conduction: Conduction in terms of band and atomic bonding models
12.7 Electrical conduction: Electron mobility
12.8 Electrical conduction: Electrical resistivity of metals
12.9 Electrical conduction: Electrical characteristics of commercial alloys
12.10 Semiconductivity: Intrinsic semiconduction
12.11 Semiconductivity: Extrinsic semiconduction
12.12 Semiconductivity: The temperature dependence of carrier concentration
12.13 Semiconductivity: Factors that affect carrier mobility
12.14 Semiconductivity: The hall effect
12.15 Semiconductivity: Semiconductor devices
12.16 Electrical conduction in ionic ceramics and in polymers: Conduction in ionic materials
12.17 Electrical conduction in ionic ceramics and in polymers: Electrical properties of polymers
12.18 Dielectric behavior: Capacitance
12.19 Dielectric behavior: Field vectors and polarization
12.20 Dielectric behavior: Types of polarization
12.21 Dielectric behavior: Frequency dependence of the dielectric constant
12.22 Dielectric behavior: Dielectric strength
12.23 Dielectric behavior: Dielectric materials
12.24 Other electrical characteristics of materials: Ferroelectricity
12.25 Other electrical characteristics of materials: Piezoelectricity
12.26 Summary
12.27 Equation summary
12.28 List of symbols
12.29 References
12.30 Questions and problems
13. Types and Applications of Materials
13.1 Introduction
13.2 Types of metal alloys: Ferrous alloys
13.3 Types of metal alloys: Nonferrous alloys
13.4 Types of ceramics: Glasses
13.5 Types of ceramics: Glass-ceramics
13.6 Types of ceramics: Clay products
13.7 Types of ceramics: Refractories
13.8 Types of ceramics: Abrasives
13.9 Types of ceramics: Cements
13.10 Types of ceramics: Ceramic biomaterials
13.11 Types of ceramics: Carbons
13.12 Types of ceramics: Advanced ceramics
13.13 Types of polymers: Plastics
13.14 Types of polymers: Elastomers
13.15 Types of polymers: Fibers
13.16 Types of polymers: Miscellaneous applications
13.17 Types of polymers: Polymeric biomaterials
13.18 Types of polymers: Advanced polymeric materials
13.19 Summary
13.20 References
13.21 Questions and problems
14. Synthesis, Fabrication, and Processing of Materials
14.1 Introduction
14.2 Fabrication of metals: Forming operations
14.3 Fabrication of metals: Casting
14.4 Fabrication of metals: Miscellaneous techniques
14.5 Fabrication of metals: 3D printing (additive manufacturing)
14.6 Thermal processing of metals: Annealing processes
14.7 Thermal processing of metals: Heat treatment of steels
14.8 Fabrication of ceramic materials: Fabrication and processing of glasses and glass-ceramics
14.9 Fabrication of ceramic materials: Fabrication and processing of clay products
14.10 Fabrication of ceramic materials: Powder pressing
14.11 Fabrication of ceramic materials: Tape casting
14.12 Fabrication of ceramic materials: 3D printing of ceramic materials
14.13 Synthesis and fabrication of polymers: Polymerization
14.14 Synthesis and fabrication of polymers: Polymer additives
14.15 Synthesis and fabrication of polymers: Forming techniques for plastics
14.16 Synthesis and fabrication of polymers: Fabrication of elastomers
14.17 Synthesis and fabrication of polymers: Fabrication of fibers and films
14.18 Synthesis and fabrication of polymers: 3D printing of polymers
14.19 Summary
14.20 References
14.21 Questions and problems
15. Composites
15.1 Introduction
15.2 Particle-reinforced composites: Large-particle composites
15.3 Particle-reinforced composites: Dispersion-strengthened composites
15.4 Fiber-reinforced composites: Influence of fiber length
15.5 Fiber-reinforced composites: Influence of fiber orientation and concentration
15.6 Fiber-reinforced composites: The fiber phase
15.7 Fiber-reinforced composites: The matrix phase
15.8 Fiber-reinforced composites: Polymer-matrix composites
15.9 Fiber-reinforced composites: Metal-matrix composites
15.10 Fiber-reinforced composites: Ceramic-matrix composites
15.11 Fiber-reinforced composites: Carbon-carbon composites
15.12 Fiber-reinforced composites: Hybrid composites
15.13 Fiber-reinforced composites: Processing of fiber-reinforced composites
15.14 Structural composites: Laminar composites
15.15 Structural composites: Sandwich panels
15.16 Structural composites: Nanocomposites
15.17 Summary
15.18 Equation summary
15.19 List of symbols
15.20 References
15.21 Questions and problems
16. Corrosion and Degradation of Materials
16.1 Introduction
16.2 Corrosion of metals: Electrochemical considerations
16.3 Corrosion of metals: Corrosion rates
16.4 Corrosion of metals: Prediction of corrosion rates
16.5 Corrosion of metals: Passivity
16.6 Corrosion of metals: Environmental effects
16.7 Corrosion of metals: Forms of corrosion
16.8 Corrosion of metals: Corrosion environments
16.9 Corrosion of metals: Corrosion prevention
16.10 Corrosion of metals: Oxidation
16.11 Degradation of polymers: Swelling and dissolution
16.12 Degradation of polymers: Bond rupture
16.13 Degradation of polymers: Weathering
16.14 Summary
16.15 Equation summary
16.16 List of symbols
16.17 References
16.18 Questions and problems
17. Thermal Properties
17.1 Introduction
17.2 Heat capacity
17.3 Thermal expansion
17.4 Thermal conductivity
17.5 Thermal stresses
17.6 Summary
17.7 Equation summary
17.8 List of symbols
17.9 References
17.10 Questions and problems
18. Magnetic Properties
18.1 Introduction
18.2 Basic concepts
18.3 Diamagnetism and paramagnetism
18.4 Ferromagnetism
18.5 Antiferromagnetism and ferrimagnetism
18.6 The influence of temperature on magnetic behavior
18.7 Domains and hysteresis
18.8 Magnetic anisotropy
18.9 Soft magnetic materials
18.10 Hard magnetic materials
18.11 Magnetic storage
18.12 Superconductivity
18.13 Summary
18.14 Equation summary
18.15 List of symbols
18.16 References
18.17 Questions and problems
19. Optical Properties
19.1 Introduction
19.2 Basic concepts: Electromagnetic radiation
19.3 Basic concepts: Light interactions with solids
19.4 Basic concepts: Atomic and electronic interactions
19.5 Optical properties of nonmetals: Refraction
19.6 Optical properties of nonmetals: Reflection
19.7 Optical properties of nonmetals: Absorption
19.8 Optical properties of nonmetals: Transmission
19.9 Optical properties of nonmetals: Color
19.10 Optical properties of nonmetals: Opacity and translucency in insulators
19.11 Applications of optical phenomena: Luminescence
19.12 Applications of optical phenomena: Photoconductivity
19.13 Applications of optical phenomena: Lasers
19.14 Applications of optical phenomena: Optical fibers in communications
19.15 Summary
19.16 Equation summary
19.17 List of symbols
19.18 References
19.19 Questions and problems
20. Environmental and Societal Issues in Materials Science and Engineering
20.1 Introduction
20.2 Environmental and societal considerations
20.3 Recycling issues in materials science and engineering
20.4 Summary
20.5 References
20.6 Questions and problems
21. Appendix A: The International System of Units (SI)
21.1 The International System of Units (SI)
22. Appendix B: Properties of Selected Engineering Materials
22.1 Properties of selected engineering materials
23. Appendix C: Costs and Relative Costs for Selected Engineering Materials
23.1 Costs and relative costs for selected engineering materials
24. Appendix D: Repeat Unit Structures for Common Polymers
24.1 Repeat unit structures for common polymers
25. Appendix E: Glass Transition and Melting Temperatures for Common Polymeric Materials
25.1 Glass transition and melting temperatures for common polymeric materials
26. Appendix F: Characteristics of Selected Elements
26.1 Characteristics of selected elements
27. Appendix G: Values of Selected Physical Constants
27.1 Values of selected physical constants
28. Appendix H: Periodic Table of the Elements
28.1 Periodic table of the elements
Same Text, More Action
Based on the 6th edition of Fundamentals of Materials Science and Engineering: An Integrated Approach, this zyVersion contains the complete text of the original book plus new interactive animations, learning questions, and challenge activities to engage the student.
- 270 dynamic animations provide insight into numerous topics
- More than 900 individual questions make up learning question sets that will help students understand topics through incremental steps, keeping students engaged and providing thorough explanations of both right and wrong answers
- Over 220 Challenge Activities (“homework problems”) provide algorithmic, auto-graded versions of the end-of-chapter problems in the original text
- Fully integrated VMSE (Virtual Materials Science and Engineering) simulations encourage students to explore the key concepts of materials science and engineering
- Test Bank questions for instructors to assign as additional assessments are also included
If you prefer a metals-first approach, with polymers and ceramics treated separately in different chapters, check out Callister’s Materials Science and Engineering: An Introduction (10e).
New! Optional Challenge Activities
As an instructor, you now have the ability to mark Challenge Activities as optional, which means you can indicate which Challenge Activities your students need to complete in order to meet your course requirements. Those marked as optional won’t contribute to point totals in reports or assignments.
Tackling Misconceptions in Materials Science:
What is a zyVersion?
zyVersions are leading print titles converted and adapted to zyBooks’ interactive learning platform, allowing for a quick and easy transition to an engaging digital experience for instructors and students.
zyBooks’ web-native content helps students visualize concepts to learn faster and more effectively than with a traditional textbook.
This zyVersion of Fundamentals of Materials Science and Engineering: An Integrated Approach (6e) benefits both students and instructors:
- Instructor benefits
- Customize your course by reorganizing existing content or adding your own content
- Continuous publication model updates your course with the latest content and technologies
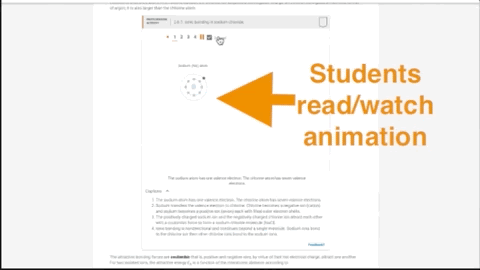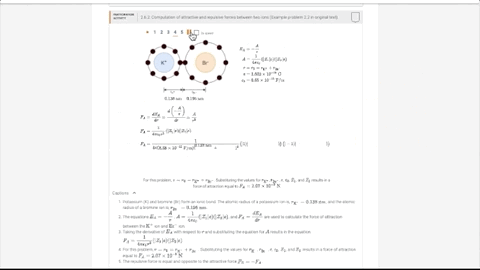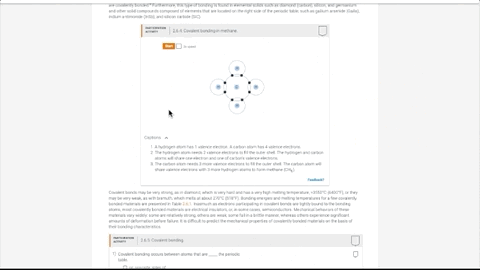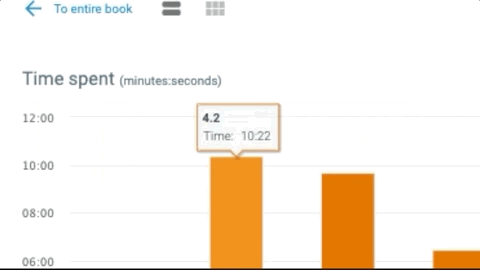
- Robust reporting gives you insight into students’ progress, reading and participation
- Student benefits
- Learning questions and other content serve as an interactive form of reading and provide instant feedback
- Concepts come to life through extensive animations embedded into the interactive content
- Save chapters as PDFs to reference material at any time, even after the course completion
Authors
William D. Callister, Jr.
Department of Metallurgical Engineering, The University of Utah
David G. Rethwisch
Department of Chemical and Biochemical Engineering, The University of Iowa
Contributors
Jim Eakins
Senior Content Developer / zyBooks
Ryan Barlow
Lead Content Developer / zyBooks